META-ANALYSIS SUGGESTS CHOOSY FEMALES GET SEXY SONS MORE THAN “GOOD GENES”
23 October 2014
Female preferences for specific male phenotypes have been documented across a wide range of animal taxa, including numerous species where males contribute only gametes to offspring production. Yet, selective pressures maintaining such preferences are among the major unknowns of evolutionary biology. Theoretical studies suggest that preferences can evolve if they confer genetic benefits in terms of increased attractiveness of sons (“Fisherian” models) or overall fitness of offspring (“good genes” models). These two types of models predict, respectively, that male attractiveness is heritable and genetically correlated with fitness. In this meta-analysis, we draw general conclusions from over two decades worth of empirical studies testing these predictions (90 studies on 55 species in total). We found evidence for heritability of male attractiveness. However, attractiveness showed no association with traits directly associated with fitness (life-history traits). Interestingly, it did show a positive correlation with physiological traits, which include immunocompetence and condition. In conclusion, our results support “Fisherian” models of preference evolution, while providing equivocal evidence for “good genes.” We pinpoint research directions that should stimulate progress in our understanding of the evolution of female choice.
Female preferences for specific, often bizarre, male phenotypes have been documented and studied across a wide range of taxa (reviewed in Andersson 1994). The evolutionary mechanisms responsible for the origin and maintenance of such preferences, however, remain a matter of controversy (reviewed by Kokko et al. 2003, 2006; Andersson and Simmons 2006; Radwan 2008). The most commonly invoked mechanism is indirect selection, which occurs when preference alleles become associated with alleles conferring high fitness to offspring (Kirkpatrick and Barton 1997).
Fisher (1930) proposed that alleles for female preferences can spread in populations because they are passed both to daughters and to sons who also inherit alleles for sexually attractive traits from their fathers. Attractive sons of choosy females enjoy higher than average reproductive success because they are preferred by choosy females. This leads to the positive feedback loop that characterizes the Fisher runaway process: female choice selects for male attractiveness traits, and the resulting gametic disequilibrium between preference and attractiveness alleles generates indirect selection for the preference (Lande 1981). The “good genes” hypothesis proposes that female preferences are subject to an additional source of indirect selection whenmale attractiveness signals genetic quality (Zahavi 1975) or more precisely, breeding value for fitness (Hunt et al. 2004; Tomkins et al. 2004). In other words, “good genes” models assume that male attractiveness depends largely on pleiotropic effects of loci controlling other aspects of fitness, such as lifehistory traits (LHTs) (Iwasa et al. 1991), resistance to parasites (Hamilton and Zuk 1982), and condition (Hunt et al. 2004; Tomkins et al. 2004). Indirect benefits to choosy females arise, because their offspring inherit high-fitness alleles in these loci from attractive fathers. Here, we employ a meta-analytical approach to evaluate empirical evidence for both the Fisherian and “good genes” models.
In 1999, Møller and Alatalo published a meta-analysis of 22 papers investigating relationships between sire attractiveness traits and offspring survival in 22 animal species. They found a weak, yet significant, positive relationship with an average correlation coefficient of 0.122 (Møller and Alatalo 1999). However, survival is only one of many fitness components, and is likely traded off against other LHTs (e.g., Bochdanovits and de Jong 2004; Blomquist 2009; Mills et al. 2010). Therefore, the relationship between male traits and offspring survival alone yields only an imprecise estimate of the relationship between male traits and offspring fitness. Taking into account a wider range of fitnessrelated traits would give a much more comprehensive view of the strength of indirect selection arising as a result of a “good genes” process. Moreover, many studies on this topic have been conducted and published since Møller and Alatalo’s work (they constitute over 60% of studies included in our dataset), and modern meta-analytical methods, which were not available in 1999, enable controlling for phylogenetic nonindependence among studies (Hadfield and Nakagawa 2010).
Our meta-analysis tests the main prediction of indirect selection models: that sexually selected traits will be associated with offspring fitness in terms of either sexual attractiveness of sons (“Fisherian” benefits) or other fitness components (“good genes” benefits). We took into account all types of fitness-related traits, including life-history, physiological, behavioral, morphological, as well as sexually selected traits. We tested for differences in effect size between different types of fitness traits.We also examined the influence of three other factors: type of sexually selected trait, sex of the individuals scored for fitness traits, and species sex chromosome system. We expected that competitive sexual traits, such as body size or dominance, may differ in the magnitude of indirect benefits from purely ornamental traits. Furthermore, due to intralocus sexual conflict, genetic benefits may be reduced, and possibly even outweighed by costs, for female progeny of highfitness males (Pischedda and Chippindale 2006). A recent model (Connallon 2010) shows that indirect effects of mating with such males may also depend on the sex chromosome system, with the benefits being smaller and the costs more pronounced in species where males are the heterogametic sex. We therefore predicted that the mean effect size would be higher for male than for female offspring, and also higher for female-heterogametic than for male-heterogametic species. Finally, we addressed the issue of differential maternal allocation, that is, correlation between female reproductive investment and the attractiveness of her mate, which may alter selection pressures on female choice (Fawcett et al. 2011) and bias the estimates of sire attractiveness offspring fitness correlations (Sheldon 2000; Horv´athov´a et al. 2011).
Methods
Data collecting and handling
We searched Web of Science using various combinations of the following keywords: sexual selection, ornament, mate choice, female choice, female preferences, good genes, genetic benefits, indirect benefits, Fisherian benefits, offspring, handicap, Hamilton and Zuk, parasite-mediated sexual selection, parasite resistance, immunocompetence, immunity, run-away selection, and heritability. Additionally,we also looked for papers citingMøller and Alatalo (1999). We collected studies reporting on either (1) genetic correlations between male sexual traits and other fitness-related characters, estimated using quantitative genetic methods (animal model, full-sibling/half-sibling designs), (2) correlations between sire sexual traits and offspring fitness-related traits, or (3) heritability of male sexual characters. In (1) and (2), we included data from species with multiple sexually selected traits, where pairs of such traits were correlated with each other. In all cases, we only took into account male sexual traits known or supposed to be targets of female choice, therefore excluding data on traits used only for intrasexual competition for mates. We also included studies where measures of male mating success (e.g., comparing males that did and did not achieve copulations in mating trials) were used instead of specific sexual traits, provided that (1) the success could be attributed to female choice rather than/apart from male– male competition and (2) different individual females were used to determine mating success of any given male and to produce his progeny scored for fitness traits. This excluded some high-profile studies (e.g., Byers and Waits 2006; Jaquiery et al. 2009; Taylor et al. 2010) but was necessary to ensure that male quality would not be confused with genetic compatibility effects.
We only included studies that satisfied the following criteria: (1) there were no major problems with statistical methodology (in particular, we excluded publications in which the analyses were based on pseudoreplication, unless we obtained additional information from the authors enabling us to calculate unbiased effect sizes); (2) it was possible to calculate effect size and determine its direction; (3) paternity had been experimentally controlled or genetically confirmed—or the frequency of extra-pair offspring was known to be <15% in the population studied (<20% if the sample size was at least 200), as such levels of extra-pair paternity should not bias the estimates of genetic parameters (Charmantier and Reale 2005). This approach permitted us to exclude potentially biased studies, while at the same time avoided unnecessary dataset restrictions.
We contacted authors in all cases when we could not determine effect sizes and/or directions based on the published information. We also solicited unpublished data by sending email requests to two widely subscribed mailing lists: EvolDir and ECOLOG, and individually to scientists studying sexual selection. However, only two unpublished studies were received this way. Our final dataset comprised 315 effect sizes extracted from 90 studies (see Table S1) with data on 55 species belonging to 46 animal genera: Alatalo et al. (1998), Arellano-Aguilar and Garcia (2008), Aspi and Hoikkala (1993), Bakker (1993), Barber et al. (2001), Boake (1985), Boake and Konigsberg (1998), Bonduriansky and Rowe (2005), Brooks (2000), Calsbeek and Bonneaud (2008), Collins et al. (1999), Connallon and Jakubowski (2009), Cothran (2008), Cotton et al. (2010), Cox and Calsbeek (2010), Day et al. (1996), Doty and Welch (2001), Edvardsson and Arnqvist (2006), Eilertsen et al. (2009), Evans et al. (2004), Fedorka and Mousseau (2004), Gromko (1987), Gustafsson et al. (1995), Hadfield et al. (2006), Head et al. (2005), Hill (1991), Hine et al. (2002), Hoefler et al. (2009), Horne and Ylonen (1998), Houde (1992), Iyengar and Eisner (1999), Jacob et al. (2010), Janhunen et al. (2011), Jia and Greenfield (1997), Karino and Haijima (2001), Kekalainen and Huuskonen et al. (2010), Kekalainen and Rudolfsen et al. (2010), Kemp and Rutowski (2007), Kleven et al. (2006), Kortet et al. (2004), Kotiaho and Puurtinen (2007), Kotiaho et al. (2001), Kraaijeveld et al. (2004), Kruczek, Zatorska (2008), Kurtz (2007), Kurtz and Sauer (1999), Lancaster et al. (2009), Leibowitz et al. (1995), Loyau and Lacroix (2010), Mills et al. (2007, 2010), Mitchell (1990), Møller and Alatalo (1999), Norry et al. (1997), Oksanen et al. (1999), Parker (2003), Petersson and Jarvi (2007), Petrie and Lipsitch (1994), Pitcher and Neff (2007), Polak et al. (2004), Price and Burley (1993), Reynolds and Gross (1992), Ritchie and Kyriacou (1994), Rudolfsen et al. (2005), Ryder and Siva-Jothy (2001), Sheldon et al. (2003), Simmons et al. (2010), Sirkia et al. (2010), Suk and Choe (2008), Szollosi et al. (2009), Tomkins and Simmons (1999), Vonschantz et al. (1994), Watson (1998), Wedekind et al. (2008a,b, 2001), Welch (2003), Welch et al. (1998), Wilcockson et al. (1995), Wilkinson, Taper (1999), and Zhou et al. (2011); see also Table S1 for more details.
Pearson’s product-moment correlation coefficientswere used as measures of effect size. If a paper did not report Pearson’s coefficient, we calculated it from other reported statistics (such as F, P, etc.) or from digitized figures. In order to be used in further analyses, each effect size was transformed to Fisher’s Z (Zr) and its associated variance was calculated as mev=1/(n−3), where n is the number of males scored for the sexually selected trait (Borenstein et al. 2009). According to Cohen’s benchmarks, Zr values of ca. 0.10, 0.31, and 0.55 correspond respectively to small, medium, and large effect size (see Horv´athov´a et al. 2011).
We accounted for several additional details of study design: (1) whether individuals scored for fitness traits originated from natural or experimentally established pairings, (2) whether direct benefits (e.g., nutrient gifts or paternal care) of mating with a particular male could influence offspring fitness or estimate thereof, or were excluded (experimentally or because the species studied has a non-resource based mating system), and (3) whether differential allocation by females into offspring, based on male attractiveness, could influence offspring fitness or was experimentally excluded (by using in vitro fertilization or artificial insemination). Based on these, we created three datasets (see Table S1): D1—full dataset (315 effect sizes from 90 studies on 55 species from 46 genera); D2—dataset including only studies in which pairs were formed experimentally (therefore excluding potentially confounding effects of nonrandom pairings) and direct male effects were excluded (212 effect sizes, 65 studies, 39 species from 31 genera); and D3—the most conservative set, consisting only of studies in which the possibility of differential allocation by females was excluded (88 effect sizes, 26 studies, 15 species from 13 genera). Meta-analyses were carried out separately on each of the three datasets.
Statistical analyses
All meta-analyses were performed using generalized linear mixed models implemented in the R package MCMCglmm (Hadfield 2010, R Development Core Team 2010). MCMCglmm fits linear models using Monte Carlo methods and Bayesian Markov chain sampling. We fitted the models without phylogeny (mixedeffects meta-analysis, MM) and with phylogeny (phylogenetic mixed-effects meta-analysis, PMM). In MM models, three random factors were specified: taxon ID, study ID, and repeat— this last factor accounting for situations where one fitness trait was correlated with multiple sexual traits, or the same pair of sex-fitness traits was analyzed for multiple (natural or experimental) populations of the same species in the same study. In PMM models, taxon ID was replaced by a random factor (called animal in MCMCglmm package) accounting for nonindependence due to shared phylogenetic history. A topology of the phylogenetic tree used for PMMs (Supplement 3 in Supporting information) was constructed based on literature data. Our full dataset included 46 vertebrate and invertebrate genera, 41 of which were represented by only one species each, whereas one (Drosophila) was represented by seven species (see Table S1). Because of such unequal clustering of species within our phylogenetic tree, we decided to base all phylogeny-corrected analyses involving D1 and D2 datasets on the tree constructed to the level of genus only. Using a more detailed tree (to the level of species) would have led to chain mixing problems in MCMC and would not have contributed significantly more phylogenetic information to our analyses. For the D3 dataset, species-level phylogenetic tree was applied, because this set did not include Drosophila and the unequal clustering problem did not apply. To enable direct comparisons between PMM and MM models, genus names were used as taxon ID in MM models fitted to D1 and D2 datasets, whereas species names were used for the D3 dataset.
Using D1 and D2 datasets, we tested the influence of four fixed factors on the magnitude of indirect benefits: type of sexually selected trait, type of fitness trait, sex of the individuals scored for a fitness trait, and species’ sex chromosome system. We classified sexually selected traits as display (purely ornamental) and competitive (size/dominance related); a third category, “other,” consisted of traits that could not be assigned to either of the two (such as nuptial gift, mating success that could not be specifically attributed to either display or dominance, or a trait increasing postcopulatory success, see Table S1). We initially classified fitness traits as life history (survival, reproductive, or growth related), sexually selected (ornamental or competitive), behavioral, morphological (adult size related), and physiological (e.g., immunocompetence or condition) (see Table S1); however, due to low sample sizes in the last three categories we combined them into one, labeled as “performance,” for the main analyses. Sex of the individuals scored for fitness traits was coded as 0 = female, 1 = both, and 2 = male, hence allowing us to test for a directional effect of sex. Sex chromosome system was classified as female heterogametic, male heterogametic, and unknown/none. Fourteen MM and 14 PMM models, including various combinations of factors and their interactions, were fitted to both D1 and D2 datasets (see Tables S2 and S3). For the D3 dataset, due to the shortage of data, only fourMMand four PMM models were fitted (Table S4). We assessed heterogeneity arising from each random factor as a proportion of total variance (i.e., the sum of all variance components in a model) associated with this factor.
For a direct comparison with Møller and Alatalo’s (1999) work, we also performed an additional analysis on a subset of D1 dataset including only survival traits. This model estimated the magnitude and significance of the mean effect size for survival, while controlling for random effects (study ID, phylogeny, and repeat).
All models were fitted with the following parameters: number of iterations (nitt): 5,000,000, burn-in period (burnin): 100,000, thinning interval (thin): 500. Flat noninformative priors with a uniform low degree of belief across all parameters were set. The best model for each dataset was chosen based on the deviance information criterion (DIC) (Hadfield 2010). The significance of study ID and phylogenetic (or genus ID) random effects was then determined for each of the three best models by comparing DIC of models with and without each of these factors.
We also tested for the presence of publication bias. We used funnel plots and Egger’s regression, whereby the intercept provides a measure of asymmetry indicative of publication bias (Egger et al. 1997). Following Horv´athov´a et al. (2011), we applied Egger’s regression to the residuals of our best-fitted models rather than to raw data, which accounts for the nonindependence among datapoints due to fixed and random factors.
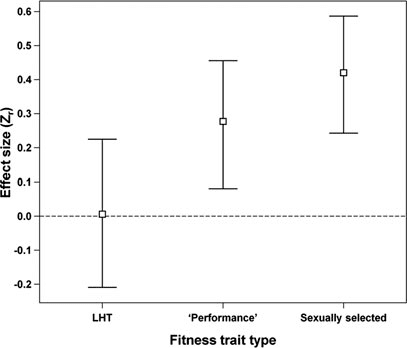
Figure 1. Mean effect sizes with confidence intervals for life history (LHT), ‘performance’ and sexually selected traits, estimated with model 12 fitted to the D1 dataset (see also Table S2).
Results
According to DIC, the PMM model with fitness trait type (Table S2, model 12) was the best of the 28 models tested on the D1 dataset (Table S2). We found that sexually selected and “performance” traits were associated with significantly greater effect sizes than LHTs; the mean for this last category was not significantly different from zero (Fig. 1, Table 1). The best model for the D2 dataset gave qualitatively identical results (see Tables S3 and S4). The mean effect size for survival traits was consistent with these for all LHTs taken together (mean Zr = 0.06, CI = −0.237 to 0.261, pMCMC = 0.633).
For the D3 dataset (consisting of studies that ruled out the influence of differential maternal allocation on the indirect benefits estimates), the PMM model with fitness trait type was best supported (model 4, Table S5), as was the case for D1 and D2 datasets. Similarly to the larger datasets, the mean effect size for LHTs was indistinguishable from zero (Table S6). The mean for “performance” traits was of a similar magnitude as in the D1 dataset (mean Zr = 0.264); however, it was not significantly different from zero (pMCMC = 0.178, Table S6). Conceivably, this could have resulted from a small sample size (only eight studies on six species from six genera). Sexually selected traits were represented here by only 2 studies (Evans 2010, Parker 2003) of 2 species (P. reticulata and G. gallus), therefore no conclusive interpretation was possible.
Table 1. Results of a phylogenetic mixed-effects meta-analysis (PMM) model including fitness trait type as an explanatory variable (model 12) fitted to the D1 dataset.
| Fixed effects: | Posterior mean | l-95% | u-95% | pMCMC | Estimated mean effect size (Zr) |
|---|---|---|---|---|---|
| Intercept (LHT) | 0.006 | −0.209 | 0.225 | 0.950 | 0.006 |
| “Performance” | 0.271 | 0.081 | 0.456 | 0.005 | 0.277 |
| Sexually selected | 0.414 | 0.243 | 0.587 | <0.001 | 0.420 |
| Random effects: | Posterior mean | Heterogeneity (%) | |||
| Study ID | 0.01 | 4.6 | |||
| Phylogeny | 0.02 | 7.8 | |||
| Repeat | 0.02 | 6.5 | |||
| Residuals | 0.25 | 81.2 |
Table 2. D1 dataset: sample sizes for different trait types.
| Fitness trait type | No. of genera | No. of studies | No. of effect sizes | |
|---|---|---|---|---|
| Life history | 31 | 49 | 145 | |
| Physiological | 9 | 11 | 43 | |
| “Performance” | Behavioral | 4 | 5 | 12 |
| Morphological | 7 | 8 | 14 | |
| Sexually selected | 24 | 38 | 101 |
Study ID and phylogenetic relationships explained little of the variance left after measurement variance was taken off (between 2.0% and 12.8%, see Tables 1, S4, and S6).
An additional analysis, performed on a subset of the D1 dataset including only data on “performance” type fitness traits, showed that mean effect size for physiological traits (Zr = 0.400) was significantly greater than zero, whereas the means for behavioral and morphological traits were not (Table S7).
Importantly, we found no evidence for publication bias in either of our datasets after controlling for random factors and fitness trait type (see Fig. S1).
Discussion
A substantial body of genetic models of sexual selection has been developed to account for the evolution of male secondary sexual traits and female preferences for particular male phenotypes (see Mead and Arnold 2004; Andersson and Simmons 2006 for reviews). Our survey returned 90 empirical studies that have explicitly tested one of the main predictions of these models: that sexually selected traits are associated with offspring fitness in terms of either sexual attractiveness of sons (“Fisherian” benefits) or other fitness components (“good genes” benefits). Although “Fisherian” and “good genes” effects can be considered extremes of the continuum of indirect genetic benefits of mate choice (Kokko et al. 2002, 2006; Radwan 2002), our meta-analysis revealed that traits associated with “Fisherian” benefits differ in effect sizes from those directly associated with fitness, that is, LHTs. Sexual traits did show a moderate and highly significant mean effect size when tested on D1 andD2 datasets, which is consistent with earlier findings of relatively high heritability of sexual traits (Pomiankowski andMøller 1995).As an important caveat, sons’ attractiveness can be influenced by the level of maternal allocation (e.g., Tschirren et al. 2012), and increased allocation by females into offspring sired by more attractive males have been observed in many taxa (Sheldon 2000; Horv´athov´a et al. 2011). Hence, it remains to be tested to what degree the observed association can be explained by genetic effects.
Strikingly, LHTs consistently showed no associations with male attractiveness, which contrasts with the results of a previous meta-analysis by Møller and Alatalo (1999), who reported a significant correlation between sire attractiveness and offspring viability. This finding appears to challenge the fundamental prediction of “good genes” hypothesis: positive correlation between sire attractiveness and offspring fitness. However, such interpretation needs to be treated with caution for two main reasons.
For one, relationship between particular LHTs and total fitness can vary with environmental conditions if optimal allocation strategy depends on environment. For example, mathematical modeling has shown that optimal growth rate is a function of external mortality, with low mortality actually favoring slower growth (Cicho´n 1997). Vast majority of studies in our dataset (36 out of 49 looking at LHTs) were carried out under laboratory conditions; therefore, it is far from obvious how the life-history parameters measured in these studies would translate to fitness in natural environment. Second, we found significant, small to moderate effects of sire ornaments on “performance”—a heterogeneous group consisting of physiological, behavioral, and morphological traits. A more detailed analysis, in which we only took into account this particular group, showed that physiological traits were associated with moderate and significant mean effect size (Table S7). It is noteworthy, as the physiological category in our dataset comprised characters related to immunocompetence and condition, which are key traits in two important “good genes” models: Hamilton–Zuk model (Hamilton and Zuk 1982) and genic capture model (Rowe and Houle 1996). Unfortunately, a very small number of studies in this group (see Table 2) does not allow for further dissection of immunocompetence versus condition effects. Moreover, the mean effect size for “performance” traits was not significantly different from zero when tested in the D3 dataset, possibly due to an even smaller sample size (eight studies only), hence it remains to be tested to what degree the observed association can be explained by genetic effects.
We expected that the magnitude of indirect benefits would also be associated with the nature of the sexually selected trait (purely ornamental vs. competitive), offspring sex (see Pischedda and Chippindale 2006), and species’ sex chromosome system (see Connallon 2010). However, models best supported according to DIC did not contain any of these factors and hence our analyses show no evidence for their influence on the indirect benefits of female choice.
To summarize, our study contrasts with the results of a previous meta-analysis (Møller and Alatalo 1999) by providing no support for “good genes” effects in terms of improved life-history parameters in offspring. Interestingly, however, we did find an indication of “good genes” effects in the form of positive correlation between sire attractiveness and offspring physiological traits such as immunocompetence and condition. Unfortunately, a small number of studies analyzing such relationships make it difficult to draw more definite conclusions.We believe that directing more research effort into analyzing genetic associations between male attractiveness, immunocompetence, and condition would provide a valuable impact into our understanding of female choice evolution. Importantly, our results support earlier evidence for Fisherian benefits of mate choice (Pomiankowski and Møller 1995). The challenge nowwill be assessing the extent towhich our estimates of these benefits are influenced by differential maternal allocation. This issue is crucial in order to estimate the strength of Fisherian indirect selection on female preferences, because only sire effects on sons’ attractiveness will benefit choosy females. We found only two papers examining Fisherian effects while controlling for maternal effects (Evans 2010 and Parker 2003)—more such studies are urgently needed.
Authors: Zofia M. Prokop, Łukasz Michalczyk, Szymon M. Drobniak, Magdalena Herdegen, and Jacek Radwan
Acknowledgments
We are grateful to J. Tomkins (University of Western Australia), N. W. Bailey (University of St. Andrews), M. Szulkin (University of Oxford), M. Gage (University of East Anglia), T. Connallon (Cornell University), J. Pearce-Duvet (Estaci´on Biol´ogica de Doˇnana), editors D. Fairbairn and E. Morrow, and two anonymous referees for their valuable comments on the manuscript. We also thank M. Stuglik for IT support, and all the people who took their time to provide unpublished data or additional information. This work was supported by a grant N N304 075835 from the Polish Ministry of Science and Higher Education to ZMP, a Natural Environment Research Council grant to ŁM, and Foundation for Polish Science (professor subsidy no. 9/2008) to JR.
Literature cited
Alatalo, R. V., J. Kotiaho, J. Mappes, and S. Parri. 1998. Mate choice for offspring performance: major benefits or minor costs? Proc. R. Soc. Lond. B 265:2297–2301.
Andersson, M. B. 1994. Sexual selection. Princeton Univ. Press, Princeton, NJ.
Andersson, M., and L. W. Simmons. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21:296–302.
Arellano-Aguilar, O., and C.M. Garcia. 2008. Exposure to pesticides impairs the expression of fish ornaments reducing the availability of attractive males. Proc. R. Soc. Lond. B. 275:1343–1350.
Aspi, J., and A. D. A. A. Hoikkala. 1993. Laboratory and natural heritabilities of male courtship song characters in Drosophila montana and D. littoralis. Heredity 70:400–406
Bakker, T. C. M. 1993. Positive genic correlation between female preference and preferred male ornament in sticklebacks. Nature 363:255–257.
Barber, I., S. A. Arnott, V. A. Braithwaite, J. Andrew, and F. A. Huntingford. 2001. Indirect fitness consequences of mate choice in sticklebacks: offspring of brighter males grow slowly but resist parasitic infections. Proc. R. Soc. Lond. B. 268:71–76.
Blomquist, G. E. 2009. Trade-off between age of first reproduction and survival in a female primate. Biol. Lett. 5:339–342.
Boake, C. R. B. 1985. Genetic consequences of mate choice: a quantitative genetic method for testing sexual selection theory. Science 227:1061–1063.
Boake, C. R. B., and L. Konigsberg. 1998. Inheritance of male courtship behavior, aggressive success, and body size in Drosophila silvestris. Evolution 52:1487–1492.
Bochdanovits, Z., and G. de Jong. 2004. Antagonistic pleiotropy for lifehistory traits at the gene expression level. Proc. R. Soc. Lond. B 271: S75–S78.
Bonduriansky, R., and L. Rowe. 2005. Intralocus sexual conflict and the genetic architecture of sexually dimorphic traits in Prochyliza xanthostoma (Diptera : Piophilidae). Evolution 59:1965–1975.
Borenstein, M., L. V. Hedges, J. P. T. Higgins, and H. R. Rothstein. 2009. Introduction to Meta-Analysis. John Wiley & Sons, Ltd., Chichester, UK.
Brooks, R. 2000. Negative genetic correlation between male sexual attractiveness and survival. Nature 406:67–70.
Byers, J. A., and L.Waits. 2006. Good genes sexual selection in nature. Proc. Natl. Acad. Sci. USA 103:16343–16345.
Calsbeek, R., and C. Bonneaud. 2008. Postcopulatory fertilization bias as a form of cryptic sexual selection. Evolution 62:1137–1148.
Charmantier, A., and D. Reale. 2005. How do misassigned paternities affect the estimation of heritability in the wild? Mol. Ecol. 14:2839–2850.
Cicho´n, M. 1997. Evolution of longevity through optimal resource allocation. Proc. R. Soc. Lond. B 264:1383–1388.
Collins, R. D., Y. Jang, K. Reinhold, andM. D. Greenfield. 1999. Quantitative genetics of ultrasonic advertisement signalling in the lesser waxmoth Achroia grisella (Lepidoptera : Pyralidae). Heredity 83:644–651.
Connallon, T. 2010. Genic capture, sex linkage, and the heritability of fitness. Am. Nat. 175:564–576.
Connallon, T., and E. Jakubowski. 2009. Association between sex ratio distortion and sexually antagonistic fitness consequences of female choice. Evolution 63:2179–2183.
Cothran, R. D. 2008. Direct and indirect fitness consequences of female choice in a crustacean. Evolution 62:1666–1675.
Cotton, S., J. Small, R. Hashim, and A. Pomiankowski. 2010. Eyespan reflects reproductive quality in wild stalk-eyed flies. Evol. Ecol. 24:83–95.
Cox, R. M., and R. Calsbeek. 2010. Cryptic sex-ratio bias provides indirect genetic benefits despite sexual conflict. Science 328:92–94.
Day, T. H., C. S. Crean, A. S. Gilburn, D. M. Shuker, and R. W. Wilcockson. 1996. Sexual selection in seaweed flies: genetic variation in male size and its reliability as an indicator in natural populations. Proc. R. Soc. Lond. B 263:1127–1134.
Doty, G. V., and A. M. Welch. 2001. Advertisement call duration indicates good genes for offspring feeding rate in gray tree frogs (Hyla versicolor). Behav. Ecol. Sociobiol. 49:150–156.
Edvardsson, M., and G. Arnqvist. 2006. No apparent indirect genetic benefits to female red flour beetles preferring males with intense copulatory courtship. Behav. Genet. 36:775–782.
Egger, M., G. D. Smith, M. Schneider, and C. Minder. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315: 629–634.
Eilertsen, E. M., B. J. Bardsen, S. Liljedal, G. Rudolfsen, and I. Folstad. 2009. Experimental evidence for paternal effects on offspring growth rate in Arctic charr (Salvelinus alpinus). Proc. R. Soc. Lond. B 276: 129–136.
Evans, J. P. 2010. Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc. R. Soc. Lond. B 277:3195–3201.
Evans, J. P., J. L. Kelley, A. Bisazza, E. Finazzo, and A. Pilastro. 2004. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. Lond. B 271:2035–2042.
Fawcett, T. W., B. Kuijper, F. J. Weissing, and I. Pen. 2011. Sex-ratio control erodes sexual selection, revealing evolutionary feedback from adaptive plasticity. PNAS 108:15925–15930.
Fedorka, K. M., and T. A. Mousseau. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429:65–67.
Fisher, R. A. 1930. The genetical theory of natural selection. Oxford Univ. Press, Oxford, U.K.
Gromko, M. H. 1987. Genetic constraint on the evolution of courtship behaviour in Drosophila melanogaster. Heredity 58:435–441.
Gustafsson, L., A. Qvarnstrom, and B. C. Sheldon. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375:311–313.
Hadfield, J. D. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33:1–22.
Hadfield, J. D., and S. Nakagawa. 2010. General quantitative methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23:494–508.
Hadfield, J. D., M. D. Burgess, A. Lord, A. B. Phillimore, S. M. Clegg, and I. P. F. Owens. 2006. Direct versus indirect sexual selection: genetic basis of colour, size and recruitment in a wild bird. Proc. R. Soc. Lond. B 273:1347–1353.
Hamilton,W. D., and M. Zuk 1982. Heritable true fitness and bright birds—a role for parasites. Science 218:384–387.
Head, M. L., J. Hunt, M. D. Jennions, and R. Brooks. 2005. The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol. 3:289–294.
Hill, G. E. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339.
Hine, E., S. Lachish, M. Higgie, and M. W. Blows. 2002. Positive genetic correlation between female preference and offspring fitness. Proc. R. Soc. Lond. B 269:2215–2219.
Hoefler, C. D., A. L. Carlascio,M. H. Persons, and A. L. Rypstra. 2009. Male courtship repeatability and potential indirect genetic benefits in a wolf spider. Anim. Behav. 78:183–188.
Horne, T. J., and H. Ylonen. 1998. Heritabilities of dominance-related traits in male bank voles (Clethrionomys glareolus). Evolution 52:894–899.
Horv´athov´a, T., S. Nakagawa, and T. Uller. 2011. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. Lond. B 279:163–170.
Houde, A. E. 1992. Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69:229–235.
Hunt, J., L. F. Bussiere, M. D. Jennions, and R. Brooks. 2004. What is genetic quality? Trends Ecol. Evol. 19:329–333.
Iwasa, Y., A. Pomiankowski, and S. Nee. 1991. The evolution of costly mate preferences. II. the ‘handicap’ principle. Evol. 45:1431–1442.
Iyengar, V. K., and T. Eisner. 1999. Heritability of body mass, a sexually selected trait, in an arctiid moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. USA 96:9169–9171.
Jacob, A., G. Evanno, B. A. von Siebenthal, C. Grossen, and C. Wedekind. 2010. Effects of different mating scenarios on embryo viability in brown trout. Mol. Ecol. 19:5296–5307.
Janhunen M., N. Peuhkuri, C. R. Primmer, I. Kolari, and J. Piironen. 2011. Does breeding ornamentation signal genetic quality in Arctic charr, Salvelinus alpinus? Evol. Biol. 381:68–78.
Jaquiery, J., T. Broquet, C. Aguilar, G. Evanno, and N. Perrin. 2010. Good genes drive female choice for mating partners in the lek-breeding European treefrog. Evolution 64:108–115.
Jia, F. Y., and M. D. Greenfield. 1997. When are good genes good? Variable outcomes of female choice in wax moths. Proc. R. Soc. Lond. B 264:1057–1063.
Karino, K., and Y. Haijima. 2001. Heritability of male secondary sexual traits in feral guppies in Japan. J. Ethol. 19:33–37.
Kekalainen, J., H. Huuskonen, M. Tuomaala, and R. Kortet. 2010. Both male and female sexual ornaments reflect offspring performance in a fish. Evolution 64:3149–3157.
Kekalainen, J., G. Rudolfsen, M. Janhunen, L. Figenschou, N. Peuhkuri, N. Tamper, and R. Kortet. 2010. Genetic and potential non-genetic benefits increase offspring fitness of polyandrous females in non-resource based mating system. BMC Evol. Biol. 10:1–9.
Kemp, D. J., and R. L. Rutowski. 2007. Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution 61:168–183.
Kirkpatrick, M., and N. H. Barton. 1997. The strength of indirect selection on female mating preferences. Proc. Natl. Acad. Sci. USA 94:1282–1286.
Kleven, O., F. Jacobsen, R. Izadnegahdar, R. J. Robertson, and J. T. Lifjeld. 2006. No evidence of paternal genetic contribution to nestling cellmediated immunity in the North American barn swallow. Anim. Behav. 71:839–845.
Kokko, H., R. Brooks, J. M. McNamara, and A. I. Houston. 2002. The sexual selection continuum. Proc. R. Soc. Lond. B 269:1331–1340.
Kokko, H., R. Brooks, M. D. Jennions, and J. Morley. 2003. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270:653–664.
Kokko, H., M. D. Jennions, and R. Brooks. 2006. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37:43–66.
Kortet, R., A. Vainikka, M. J. Rantala, J. Myntti, and J. Taskinen. 2004. In vitro embryo survival and early viability of larvae in relation to male sexual ornaments and parasite resistance in roach, Rutilus rutilus L. J. Evol. Biol. 17:1337–1344.
Kotiaho, J. S., and M. Puurtinen. 2007. Mate choice for indirect genetic benefits: scrutiny of the current paradigm. Funct. Ecol. 21: 638–644.
Kotiaho, J. S., L.W. Simmons, and J. L. Tomkins. 2001. Towards a resolution of the lek paradox. Nature 410:684–686.
Kraaijeveld, K., P. J. Carew, T. Billing, G. J. Adcock, and R. A. Mulder. 2004. Extra-pair paternity does not result in differential sexual selection in the mutually ornamented black swan (Cygnus atratus). Mol. Ecol.
13:1625–1633.
Kruczek, M., and M. Zatorska. 2008. Male rank affects reproductive success and offspring performance in bank voles. Physiol. Behav. 94:611–615.
Kurtz, J. 2007. The correlation between immunocompetence and an ornament trait changes over lifetime in Panorpa vulgaris scorpionflies. Zoology 110:336–343.
Kurtz, J., and K. P. Sauer. 1999. The immunocompetence handicap hypothesis: testing the genetic predictions. Proc. R. Soc. Lond. B 266:2515–2522.
Lancaster, L. T., C. A. Hipsley, and B. Sinervo. 2009. Female choice for optimal combinations of multiple male display traits increases offspring survival. Behav. Ecol. 20:993–999.
Lande, R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl. Acad. Sci. USA 78:3721–3725.
Leibowitz, A., M. Santos, and A. Fontdevila. 1995. Heritability and selection on body size in a natural population of Drosophila buzzatii. Genetics 141:181–189.
Loyau, A., and F. Lacroix. 2010. Watching sexy displays improves hatching success and offspring growth through maternal allocation. Proc. R. Soc. Lond. B 277:3453–3460.
Mead, L. S., and S. J. Arnold. 2004. Quantitative genetic models of sexual selection. Trends Ecol. Evol. 19:264–271.
Mills, S. C., R. V. Alatalo, E. Koskela, J. Mappes, T. Mappes, and T. A. Oksanen. 2007. Signal reliability compromised by genotype-by-environment interaction and potential mechanisms for its preservation. Evolution 61:1748–1757.
Mills, S. C., A. Grapputo, I. Jokinen, E. Koskela, T. Mappes, and T. Poikonen. 2010. Fitness trade-offsmediated by immunosuppression costs in a small mammal. Evolution 64:166–179.
Mitchell, S. L. 1990. The mating system genetically affects offspring performance in woodhouse’s toad (Bufo woodhousei). Evolution 44:502–519.
Møller, A., and R. Alatalo. 1999. Good-genes effects in sexual selection. Proc. R. Soc. Lond. B 266:85–91.
Norry, F. M., J. C. Vilardi, and E. Hasson. 1997. Genetic and phenotypic correlations among size-related traits, and heritability variation between body parts in Drosophila buzzatii. Genetica 101:131–139.
Oksanen, T. A., R. V. Alatalo, T. J. Horne, E. Koskela, J. Mappes, and T. Mappes. 1999. Maternal effort and male quality in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 266:1495–1499.
Parker, T. H. 2003. Genetic benefits of mate choice separated from differential maternal investment in red junglefowl (Gallus gallus). Evolution 57:2157–2165.
Petersson, E., and T. Jarvi. 2007. Characteristics of brown trout males influence growth and survival of their offspring. J. Fish. Biol. 71:493–509.
Petrie, M., and M. Lipsitch. 1994.Avian polygyny ismost likely in populations with high variability in heritable male fitness. Proc. R. Soc. Lond. B 256:275–280.
Pischedda, A., and A. K. Chippindale. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4:2099–2103.
Pitcher, T. E., and B. D. Neff. 2007. Genetic quality and offspring performance in Chinook salmon: implications for supportive breeding. Conserv. Genet. 8:607–616.
Polak, M., W. T. Starmer, and L. L. Wolf. 2004. Sexual selection for size and symmetry in a diversifying secondary sexual character in Drosophila bipectinata duda (Diptera : Drosophilidae). Evolution 58: 597–607.
Pomiankowski, A., and A. P. Møller. 1995. A resolution of the lek paradox. Proc. R. Soc. Lond. B 260:21–29.
Price, D. K., and N. T. Burley. 1993. Constraints on the evolution of attractive traits—genetic (co)variance of zebra finch bill color. Heredity 71: 405–412.
R Development Core Team. 2010. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/5.
Radwan, J. 2002. Good genes go fisherian. Trends Ecol. Evol. 17:539–539. ———.2008. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134:113–127.
Reynolds, J. D., and M. R. Gross. 1992. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc.R. Soc. Lond. B 250:57–62.
Ritchie, M. G., and C. P. Kyriacou. 1994. Genetic variability of courtship song in a population of Drosophila melanogaster. Anim. Behav. 48:425–434.
Rowe, L., and D. Houle 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263:1415–1421.
Rudolfsen, G., L. Figenschou, I. Folstad, J. T. Nordeide, and E. Soreng. 2005. Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). J. Evol. Biol. 18:172–179.
Ryder, J. J., and M. T. Siva-Jothy 2001. Quantitative genetics of immune function and body size in the house cricket, Acheta domesticus. J. Evol. Biol. 144:646–653.
Sheldon, B. C. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15:397–402.
Sheldon, B. C., H. Arponen, A. Laurila, P. A. Crochet, and J. Merila. 2003. Sire coloration influences offspring survival under predation risk in the moorfrog. J. Evol. Biol. 16:1288–1295.
Simmons, L. W., R. M. Tinghitella, and M. Zuk. 2010. Quantitative genetic variation in courtship song and its covariation with immune function and sperm quality in the field cricket Teleogryllus oceanicus. Behav. Ecol. 216:1330–1336.
Sirkia, P. M., M. Virolainen, and T. Laaksonen. 2010. Melanin coloration has temperature-dependent effects on breeding performance that may maintain phenotypic variation in a passerine bird. J. Evol. Biol. 23: 2385–2396.
Suk, H. Y., and J. C. Choe. 2008. Dynamic female preference for multiple signals in Rhinogobius brunneus. Behav. Ecol. Sociobiol. 62: 945–951.
Szollosi, E., B. Rosivall, D. Hasselquist, and J. Torok. 2009. The effect of parental quality and malaria infection on nestling performance in the Collared Flycatcher (Ficedula albicollis). J. Ornithol. 150: 519–527.
Taylor,M. L., N.Wedell, and D. J. Hosken. 2010. Attractive males do not sire superior daughters. Evol. Ecol. 24:195–205.
Tomkins, J. L., and L.W. Simmons. 1999. Heritability of size but not symmetry in a sexually selected trait chosen by female earwigs. Heredity 82: 151–157.
Tomkins, J. L., J. Radwan, J. S. Kotiaho, and T. Tregenza. 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19:323–328.
Tschirren, B., E. Postma, A. N. Rutstein, and S. C. Griffith. 2012. When mothers make sons sexy: maternal effects contribute to the increased sexual attractiveness of extra-pair offspring. Proc. R. Soc. Lond. B279:1233–1240.
von Schantz, T., M. Grahn, and G. Goransson. 1994. Intersexual selection and reproductive success in the pheasant Phasianus colchicus. Am. Nat. 144:510–527.
Watson, P. J. 1998. Multi-male mating and female choice increase offspring growth in the spider Neriene litigiosa (Linyphiidae). Anim. Behav. 55:387–403.
Wedekind, C., R. Muller, and H. Spicher. 2001. Potential genetic benefits of mate selection in whitefish. J. Evol. Biol. 14:980–986.
Wedekind, C., G. Evanno, D. Urbach, A. Jacob, and R.Muller. 2008a. ‘Goodgenes’ and ‘compatible-genes’ effects in an Alpine whitefish and the information content of breeding tubercles over the course of the spawning season. Genetica 132:199–208.
Wedekind, C., A. Jacob, G. Evanno, S. Nussl´e, and R. Muller. 2008b. Viability of brown trout embryos positively linked to melanin-based but negatively to carotenoid-based colours of their fathers. Proc. R. Soc. Lond. B 275:1737–1744.
Welch, A. M. 2003. Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57:883–893.
Welch, A. M., R. D. Semlitsch, and H. C. Gerhardt. 1998. Call duration as an indicator of genetic quality in male gray tree frogs. Science 280: 1928–1930.
Wilcockson, R.W., C. S. Crean, and T. H. Day. 1995. Heritability of a sexually selected character expressed in both sexes. Nature 374:158–159.
Wilkinson, G., and M. Taper. 1999. Evolution of genetic variation for condition-dependent traits in stalk-eyed flies. Proc. R. Soc. Lond. B 266:1685–1690.
Zahavi, A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53:205–214.
Zhou, Y., J. K. Kelly, and M. D. Greenfield. 2011. Testing the fisherian mechanism: examining the genetic correlation between male song and female response in waxmoths. Evol. Ecol. 25:307–329.
Associate Editor: E. Morrow
Supporting Information
The following supporting information is available for this article:
Figure S1. A funnel plot (effect sizes plotted against their measures of precision, i.e., inverse standard errors) of residuals from model 12 fitted to the full dataset (D1).
Table S1. Dataset for the meta-analysis.
Table S2. The D1 (full) dataset: meta-analytical models, their compositions, and deviance information criteria (DIC).
Table S3. The D2 dataset (nonrandom pairing and direct benefits excluded): meta-analytical models, their compositions, and deviance information criteria (DIC).
Table S4. Results of the PMM model including fitness trait type as an explanatory variable (model 12) fitted to the D2 dataset.
Table S5. The D3 dataset (nonrandom pairing, direct benefits, and differential allocation excluded): meta-analytical models, their compositions, and deviance information criteria (DIC).
Table S6. Results of the PMM model including fitness trait type as an explanatory variable (model 4) fitted to the D3 dataset.
Table S7. Results of the PMM model testing three trait types grouped together as “performance.”
Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.